Whilst January saw the Earth pass through its orbital perihelion point (06:34 UTC on the 3rd Jan), we will note February as the birth-month of some notable historical French astronomers. These include:
Pierre Jules Janssen (22nd Feb, 1824), famous for his works on spectroscopy; the discovery of helium; and the founder of the Meudon Observatory;
Benjamin Baillaud (14th Feb 1848), a contemporary of the great Francois Felix Tisserand and the latter’s successor as director of the Toulouse Observatory; and
Claude Pouillet (16th Feb 1790), whom as we outlined in our blog of January 2017, made the first scientific measurement of the solar irradiance using the pyrheliometer which he designed and built.
And of interest to our work on the Limousin asteroid impact, February also marks the birthdate of Pierre Bouguer (10th Feb, 1698). Bouguer is most famous for his work on gravity and the very small changes in the Earth’s gravity due to variance in local mass. Bouguer maps are named in his honour.
Each of these holds an interest to us here at Observatoire Solaire. The life and works of both Janssen and Baillaud feature within in a compendium of short biographies we are currently preparing, and which we aim to make available in early summer (in time for summer holiday ventures!)
This month we look at a rather intriguing question. Why, considering that the Universe is made up of 98% of Hydrogen and Helium, do we have neither of these elements within our atmosphere?
En janvier la Terre est passé par le périhélie (06.34 le 3 janvier) et le mois de février marque l’anniversaire de plusieurs astronomes français comme par exemple:
Pierre Jules Janssen (22/2/1848), célèbre pour son travail sur la spectroscopie, la découverte du hélium et la fondateur de l’Observatoire de Meudon
Benjamin Baillaud, contemporain de François Félix Tisserand et son successeur comme directeur de l’Observatoire de Toulouse.
Claude Pouillet (16/2/1790) dont nous avons parlé dans notre blog de janvier 2017 et qui est le premier à mesurer scientifiquement l’irradiance solaire en utilisant le pyrhéliomètre qu’il a conçu et construit.
Le mois de février est aussi l’anniversaire de Pierre Bouguer (10/2/1698). Bouguer est célèbre pour son travail sur la gravité les cartes gravimétriques de Bouguer.
Janssen et Baillaud nous intéressent beaucoup et nous espérons les inclure dans un recueil de courtes biographies qui est en préparation et sera disponible en été.
Ce mois nous allons considérer une question fascinante: pourquoi n’y a-t-il pas d’Hydrogène et d’Hélium dans l’atomosphère?
The Universe is made up of mostly hydrogen and helium (74% and 24% by mass respectively; and more locally to our solar system 71% and 27%). However, the Earth’s atmosphere contains close to zero of both elements. Our planet’s atmosphere comprises mostly nitrogen, N2, and oxygen, O2 (78% and 20.9% by volume respectively). There is a very small amount of carbon dioxide, CO2 (0.04%) and a slightly larger amount of argon (0.9%).
(Atmospheric water varies from ~0 to 4% depending upon time and location). Photograph by kind permission of Colin Taylor
We saw in our blog of September 2017, the (nearly) Planck distribution of energy from an astronomical object. The energy distribution is the thermal emission of radiation from a heated body and originates in the atomic elements and molecules motions. All atoms and molecules have kinetic energy (thermal motion) and what we feel as temperature is the rate at which the molecules are moving. A hot object has greater thermal energy than a cooler object.
The temperature at which all molecular motion ceases is called ‘absolute-zero’. This temperature is at minus 273.15 Celsius. In astronomy and physics temperature is measured in units of Kelvin. A change in temperature of one Kelvin is the same as a change of one degree Celsius. The Kelvin scale is an absolute scale, whereas the Centigrade temperature scale was/is a relative scale based upon the triple point (freezing point) and boiling point of water.
(In the above, we have not talked about Quantum mechanical motion which is retained at absolute zero. The interested reader to referred to research topics such as Bose-Einstein condensates in ‘further-reading' [1] or [3])
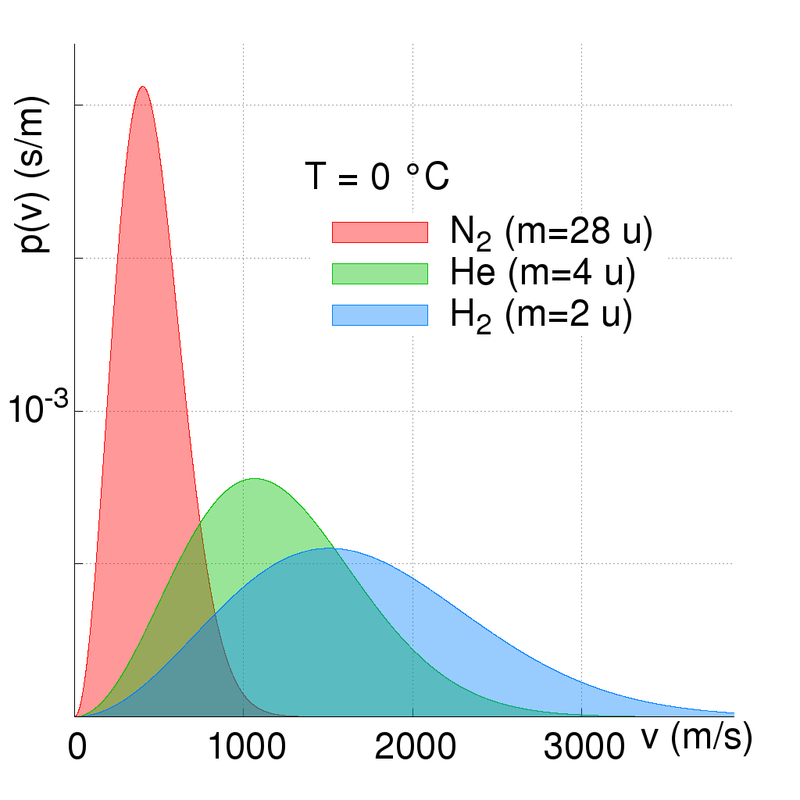
Atomic elements and molecules are all moving. However, the speed at which they move depend upon their energies (kinetic temperature) and their atomic mass. The lighter (lower mass) the element or molecule is, then the faster is its kinetic motion. The kinetic velocity of the elements is high from a human perspective; hundreds of metres per second.
If an atom or molecule within the atmosphere exceeds the Earth’s escape velocity then that matter may escape from the Earth’s gravitational field and be ‘lost’ into space. The escape velocity of any planet, or star, depends only on the planet/star’s mass. The Earth’s escape velocity is 11.2 km/second, approximately 25,000 miles per hour, at its surface. (The escape velocity reduces with radial distance from the planet’s centre of mass. We will explain this when we consider gravity in a future blog-series.)
This means for example any rocket or planetary probe needs to be accelerated to the escape velocity to be placed in a hyperbolic orbit, (i.e. open / ‘escape’) as far as the Earth is concerned. Any lower speed and the planetary probe would remain in an elliptical orbit around the Earth. The atmosphere behaves in exactly the same way. It is bound to the Earth, and retained, by gravity.
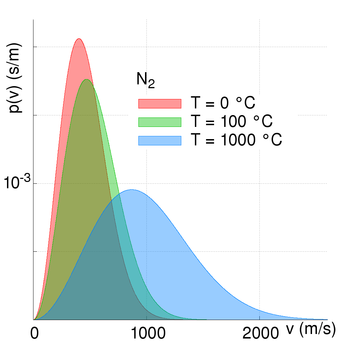
The Earth has retained a nitrogen and oxygen based atmosphere because these elements have relatively slow kinetic motions compared to that of the Earth’s escape velocity. We have no hydrogen or helium primarily because the Earth’s atmosphere is warm (we are relatively close to the Sun) and so these elements are moving fast, and some atoms can approach and exceed the escape velocity. The speed/velocity of atoms and molecules does however vary...
The Maxwell-Boltzmann distribution law for molecular velocities (strictly molecular momenta) describes the maths and physics of the kinetic motions and gives a probability function based on an average molecular velocity. The mathematically astute and interested reader is referred to further reading [2] or [3]. In numerical summary; at a temperature of zero degrees Celsius (273.15 Kelvin) the following mean molecular velocities are seen:
Hydrogen (H2) 1694
Helium (He) 1202
Nitrogen (N2) 454
Oxygen (O2) 425
Carbon Dioxide (CO2) 362
Whilst these velocities are lower than the Earth's escape velocity, three important factors come into play:-
- The first is that the table above shows average molecular velocities and there is a finite probability of velocities exceeded any specific value (i.e. the escape velocity). And at higher temperatures the molecular speed is higher (not all the Earth's atmosphere is at 273 Kelvin!) and at higher altitudes the escape velocity is lower than at sea level;
- Secondly, both mixing and stratification (separation) occurs within the atmosphere. The lighter (less dense) elements in particular are affected by these processes - heavier elements tend to 'sink' towards the lower levels as they have greater mass. A good example of this is the observation that a helium ballon rises in the air. It is less massive (and less dense) than the surrounding air;
- A tertiary reason for the lack of hydrogen in our atmosphere is that it is very reactive, and so any hydrogen in the Earth's primordial atmosphere would have combined with other elements (especially with oxygen, to form water).
So these are the reasons we're rather out of step with most of the Universe!
(In ascending level of technical complexity)
[1] The New Quantum Universe. Tony Hey and Patrick Walters. 2003
http://www.cambridge.org/gb/academic/subjects/physics/quantum-physics-quantum-information-and-quantum-computation/new-quantum-universe-2nd-edition?format=PB#RzzmezMoZYExcsGu.97
[2] The Maxwell-Boltzmann distribution
https://de.wikipedia.org/wiki/Maxwell-Boltzmann-Verteilung
[3] Atomic Astrophysics and Spectroscopy. Anil. K . Pradhan & Sultana. N. Nahar. 2011
http://www.cambridge.org/gb/academic/subjects/physics/astrophysics/atomic-astrophysics-and-spectroscopy?format=PB#x4PmHYITVvDa7Pt7.97
Next Month
Next month we will look at the overall structure of the Sun. Identifying the various layers, we will start to look at the outer-most layer of the Sun's atmosphere (the Corona) and how this plays a pivotal role in the Solar Wind. and 'space-weather'.
Next month’s blog will be issued on Saturday 24th February.